In 2020, China's domestic new drug ind declaration will reach a new record. Although the global epidemic has brought many difficulties, it has not delayed the enthusiasm and pace of domestic new drug development. So, how many imported varieties parallel to the registration of class 1 ind of domestic self-developed chemical drugs will declare ind in 2020? What is the overall trend? Is there any reference significance for domestic ind varieties? Look at this article.
Application trend of import chemical drug ind varieties in 2016-2020
Since the reform of China's new registration and classification of chemical drugs in 2016, the work of variety registration and declaration has already completed the transition and tends to be stable. For imported varieties, in addition to the declaration according to category 5.1 of the new registration classification, another important direction is the registration and declaration according to category 1 new drugs from ind.
According to the data query of pharmaceutics intelligence, in 2016, there were only 27 ind declaration acceptance numbers of imported chemical drugs in China; after two years of transition, the average annual acceptance number of 2017 / 2018 was about 80; in 2019, it doubled to 170 acceptance numbers; in 2020, the number of ind declaration of imported chemical drugs was 174, reaching a stable level again in recent two years.
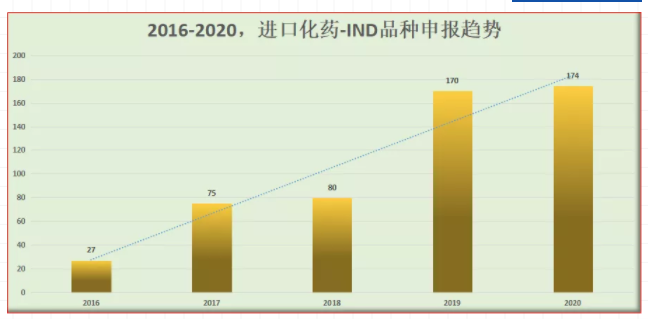
Acceptance status of imported chemical drug ind varieties in 2020
In 2020, imported chemical drugs will be registered and applied for ind in China, and the foreign well-known pharmaceutical companies with more acceptance numbers are Roche, Novartis, Pfizer, Johnson & Johnson, Bayer, Sanofi, Takeda, etc.
The representative domestic pharmaceutical companies are Baiji Shenzhou, Xinda biology, zaiding medicine, Diemai biology, Hangzhou Arnold, Deqi medicine, etc. The main information is shown in the table below.
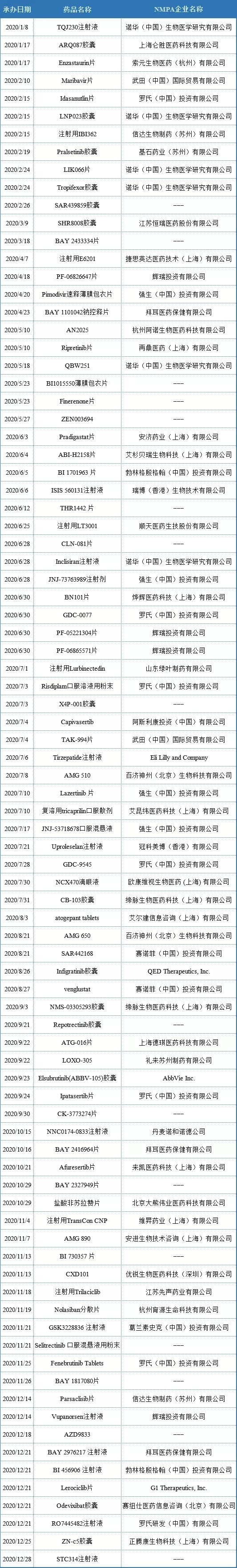
Examples of import declaration ind for several varieties of large foreign pharmaceutical companies
The product R & D pipeline of multinational pharmaceutical companies has always been the focus of domestic pharmaceutical companies; through the status of these companies registering and applying for class 1 new drug ind in China, we can better know the development rhythm of their key varieties; now, the application and registration of ind varieties of large foreign pharmaceutical companies in China in 2020 are introduced as follows.
Roche
In 2020, Roche (Roche (China) Investment Co., Ltd.) has registered 7 varieties in China by means of imported chemical drug ind, namely idasanutlin tablets (MDM2-p53 inhibitor), gdc-0077 (PI3K α inhibitor), risdiplam powder for oral solution (SMN2 splicing modifier), gdc-9545 (ER antagonist), ipatasertib tablets (PI3K / Akt pathway inhibitor), fenebrutinib Tablets (Btk inhibitor), ro7445482 injection.
Novartis
In 2020, Novartis (Novartis (China) biomedical research Co., Ltd.) has 5 varieties registered in China in the form of imported chemical drug ind, namely lnp023 capsule (factor B inhibitor), lik066 tablets (SGLT inhibitor), tropifexor capsule (FXR agonist), qbw251 (CFTR regulator) and inclisiran injection (PCSK9 targeted RNAi drug).
Pfizer
In 2020, Pfizer (Pfizer Investment Co., Ltd.) will have 4 kinds of products registered in China in the form of imported chemical drug ind, namely pf-06826647 tablets (Tyk2 inhibitor), pf-05221304 tablets (ACC inhibitor), pf-06865571 tablets (dgat2 inhibitor) and vupanorsen injection (antisense therapy).
Johnson
In 2020, Johnson & Johnson (Johnson & Johnson (China) Investment Co., Ltd.) has 4 varieties registered in China in the form of imported chemical drug ind, namely pimodivir rapid release film coated tablets (influenza A virus polymerase inhibitor), jnj-73763989 injection (RNAi therapy), lazertinib tablets (EGFR TKIs) and jnj-53718678 oral suspension (fusion protein inhibitor).
Bayer
In 2020, Bayer (Bayer healthcare Co., Ltd.) will register three kinds of products in the form of imported chemical drug ind in China, namely bay110042 sodium controlled release tablets (SGC agonist), bay2416964 tablets (AHR inhibitor) and bay2976217 injection.
Sanofi
In 2020, Sanofi (Sanofi (China) Investment Co., Ltd.) has two kinds of products registered in China in the form of imported chemical drug ind, namely sar442168 (Btk inhibitor) and winglustat (GCS inhibitor).
Takeda
In 2020, Wutian (China) International Trade Co., Ltd. will register two kinds of products in China in the form of imported chemical drug ind, namely Maribavir tablets (antiviral therapy) and tak-994 tablets (ox2r agonist).
Summary
The above is the general situation of "chemical drug class 1" import ind accepted varieties in 2020. Through the comparison of the number of registration and declaration over the years, it is not difficult to see that foreign pharmaceutical companies attach importance to China's drug market, but also can see that China's drug audit department has strict standards for all varieties. And further compare the ind declaration status of imported varieties and domestic pharmaceutical enterprises (see "inventory in 2020: 164 first ind of" class 1 new drugs ", Zhengda Tianqing, dongyangguang drugs...") It can be seen that there is a gap between China and foreign countries in the innovation level of ind varieties. In addition, domestic pharmaceutical enterprises have introduced and developed many key varieties through the way of license in, and are also in line with the innovation frontier. But, after all, we still need to develop our own FIC varieties, or need real independent innovation, so as to really meet our own urgent clinical needs.




