Recently, Kelun Pharmaceutical announced the Phase I clinical data of SKB264 at the CSCO annual meeting. As of April 28, 2021, 17 patients have received at least one efficacy evaluation. The total remission rate of SKB264 reached 41.2% (7/17), and the disease control rate (DCR) reached 70.6%. Among them, 2 of 5 triple-negative breast cancer patients achieved partial remission (ORR=40%), and 3 of 5 ovarian cancer patients achieved partial remission (ORR=60%). In terms of safety, adverse events above grade 3 mainly included decreased neutrophil count (27.78%, 5/18), decreased white blood cell count (22.22%, 4/18) and anemia (16.67%, 3/18). In general, SKB264 has shown good efficacy and safety in patients with solid tumors, but the current sample size is small, and the support of subsequent data is still needed. SKB264 is a TROP2 ADC, which is the key variety of Kronbote ADC platform. As a popular track in the ADC field, TROP2 has attracted many companies in recent years, including Genting Xinyao, Kelun Pharmaceutical, Junshi Biology, Shijian Biology, etc. Daiichi Sankyo/AstraZeneca, a leading company in the ADC field, has also been deeply involved in the TROP2 track. The global R&D of DS-1062 is already in key clinical trials and was approved for clinical trials in China on July 21 this year. TROP2 ADC's domestic war is about to start. This article will take a closer look at the competitive landscape.
(1) Analysis of Trop2
Trop2 is a cell surface glycoprotein encoded and expressed by the TACSTD 2 gene, which is closely related to the occurrence and development of tumors. Studies have found that in different tissues of the human body, the expression level of Trop2 is different, and the breast gland has the highest expression level. In tumor cells, the expression of Trop2 is significantly increased, and it promotes tumor cell growth, proliferation and metastasis by regulating calcium ion signaling pathway, cyclin expression and reducing fibronectin adhesion. In addition, TROP2 can also interact with β-catenin in the Wnt signaling cascade, and thus plays a role in the transcription of nuclear oncogenes and cell proliferation. In general, the high expression of Trop2 is closely related to the proliferation, invasion, metastasis and spread of cancer cells, resulting in a significant reduction in the survival time of cancer patients.

Figure: TROP2 mechanism of action
(2) Trodelvy: Take the lead in listing
Trodelvy (Sacituzumab Govitecan, IMMU-132) is the world's first Trop2 ADC drug on the market, originally developed as Immunomedics. Trodelvy was approved for marketing by the FDA in 2020, and its indication is triple-negative breast cancer (TNBC). In April 2021, Trodelvy was again approved for urothelial cancer indications. According to Gilead’s financial report, in 2020, Trodelvy will achieve sales revenue of $49 million. From the structural point of view, Trodelvy is composed of three parts, namely, human monoclonal antibody Sacituzumab targeting Trop2, maleimide linker and topoisomerase I inhibitor SN-38, with a DAR equal to 7.4. In terms of structure, the use of SN-38 is very eye-catching. SN-38 is the active metabolite of irinotecan. It is a moderately toxic DNA topoisomerase inhibitor, which effectively circumvents the deficiencies of the narrow anti-tumor spectrum of microtubule inhibitors. Through the design of medium toxin and high DAR, Trodelvy not only maintains high anti-tumor efficacy, but also effectively reduces the off-target toxicity of the drug, taking into account the efficacy and safety of the drug.
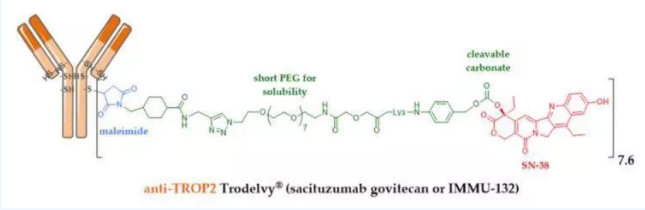
Figure: Trodelvy structure
Data source: Immunomedics
As the first Trop2 ADC drug on the market, Trodelvy has excellent clinical data. In the ASCENT study, the trial included 108 TNBC patients who had previously received at least two standard treatment progressions. Trodelvy achieved an objective response rate of 33.3%, with CR of 2.8%, mDoR of 7.7 months, and mPFS of 5.5 months. The approval of the indication for urothelial carcinoma is based on the international phase 2 single-arm TROPHY study. Among 112 evaluable patients, Trodelvy achieved an objective response rate of 27.7%, of which the complete response rate was 5.4% and the partial response rate was 22.3% , MDoR is 7.2 months. In China, the R&D and commercialization rights of Trodelvy belong to Genting Shinyao. In May 2021, NMPA has accepted Trodelvy's application for marketing authorization for biological products, and the indication is the third-line treatment of metastatic triple-negative breast cancer. Trodelvy is expected to be approved for listing by the end of 2021.
(3) DS-1062: The veteran is out
DS-1062 is a Trop2 ADC drug jointly developed by Daiichi Sankyo and AstraZeneca. DS-1062 consists of three parts: human IgG1 monoclonal antibody Datopotamab targeting Trop2, cleavable cysteine linker and DNA topoisomerase DXd. DS-1062 has a DAR of 4. As a pioneer and leader in the ADC field, Daiichi Sankyo has continued to use creative ideas to improve the development of ADC drugs. Like the famous DS-8201, DS-1062 also uses DXd. As an innovative DNA topoisomerase inhibitor, DXd has ten times the activity of irinotecan (SN-38), which can interfere with DNA replication, recombination and gene expression. From the payload point of view, compared with Trodelvy, DS-1062's small molecule drugs are more toxic and have better killing ability on target cells. In addition, DS-1062 did not follow the design of DS-8201 ultra-high DAR, but controlled the DAR at 4. In fact, the scientific community has always questioned the high DAR. High DAR may weaken the uniformity of the drug and cause higher toxic side effects. Controlling the DAR at the level of 4 can effectively achieve a balance between drug efficacy and safety.

Picture: DS-1062 structure
Data source: Daiichi Sankyo
Currently, DS-1062 focuses on non-small cell lung cancer and triple-negative breast cancer indications. On 2020WCLC, Daiichi Sankyo disclosed the results of a phase I clinical study of DS-1062 in the treatment of advanced NSCLC. 175 patients with advanced NSCLC who failed the previous standard treatments (including targeted drugs, PD-1/L1, chemotherapy, etc.) received three dose levels of DS-1062 single-drug (4mg/kg in 50 cases, 6mg/kg in 45 cases, 8mg /kg (80 cases), the 6mg/kg group achieved 21% ORR, DCR 67%, and PFS for 8.2 months. At the ESMO breast cancer online conference in 2021, researchers announced the Phase I clinical data of DS-1062 in the treatment of TNBC. Among 21 evaluable patients, the initial objective response rate of DS-1062 reached 43%, and 5 of them achieved complete or partial remission, with DCR reaching 95%. It is worth mentioning that the study also included 2 patients who had previously received Trodelvy and had disease progression. In general, DS-1062 has the potential to surpass Trodelvy for TNBC indications. However, considering the current limited number of sample populations, more trial populations need to be included in the follow-up to further determine the efficacy of the drug.
(4) Domestic Trop2 ADC layout: separate rule of the group
Considering the therapeutic prospects of Trop2 for breast cancer, urothelial cancer and non-small cell lung cancer, there are also a number of domestic companies focusing on this target. In addition to Genting Xinyao and Kelun Pharmaceutical, the Trop2 ADCs of Junshi Biological and Shijian Biological are in the clinical stage. Fudan Zhangjiang’s has submitted an IND, and CSPC, Luoqi Biological, Hausen Pharmaceutical, etc. also have Trop2. Antibody patent, the commercialization rights of the Trop2 antibody of Fuhong Henlius license in Chiome Japan's China region. Junshi Bio/Duoxi Bio's JS108 is a recombinant humanized anti-Trop2 monoclonal antibody-Tub196 coupling agent for injection and is currently in clinical phase I. On July 21, 2020, JS108 was approved and issued by the NMPA "Drug Clinical Trial Approval Notice" for clinical trials of advanced solid malignant tumors. On November 25, 2020, Shanghai Junshi Biologics Phase 1 clinical study of JS108 has completed the first patient administration. Shijian Bio’s ESG-401 is a recombinant humanized anti-Trop2 monoclonal antibody-SN38 conjugate. The conjugate uses an innovative highly stable and degradable linker, which releases very little free toxins in the circulation. Tumor tissue is highly enriched and quickly endocytosed, thereby effectively killing tumor cells and inhibiting tumor growth. In a series of pre-clinical studies, ESG-401 showed excellent safety. In the safety evaluation of non-human primate GLP with high-dose repeated administration, no off-target toxicity and toxicity outside of tumor tissue were observed. On July 21, 2021, ESG-401 was approved for clinical use.
(5) Summary
The high expression of Trop2 is closely related to the proliferation, invasion and metastasis of cancer cells. As a broad-spectrum tumor marker, Trop2 has a broad market prospect. In recent years, the research and development of Trop2 ADC drugs has progressed rapidly. Trodelvy has successfully passed the FDA approval in 2020 and achieved sales of 49 million US dollars that year. Entering 2021, Trodelvy is again approved for urothelial cancer indications, which is expected to further expand sales. Focusing on the domestic market, Genting Xinyao, Kelun Pharmaceutical, Junshi Bio, and Shijian Bio are all deeply involved in Trop2 ADC. Genting Xinyao has submitted a listing application and is expected to be the first to be approved for listing. However, behind the lively R&D, we must be alert to the risks of homogenization and R&D failure. The R&D of dozens of companies may lead to fierce competition in the future commercialization stage, and Biotech’s Trop2 ADC has already collapsed.





