Two new drugs are included in the proposed priority review varieties! From Hengrui Pharmaceutical, Jinsai Pharmaceutical
February 21, 2022
On October 12, according to the announcement of the Center for Drug Evaluation (CDE) of the National Medical Products Administration, two new drug listing applications were planned to be included in the priority review and approval, including Hengrui Medicine’s "Cyclophosphamide Capsules" and Jinsai Pharmaceutical’s "Recombinant Human Growth Hormone."
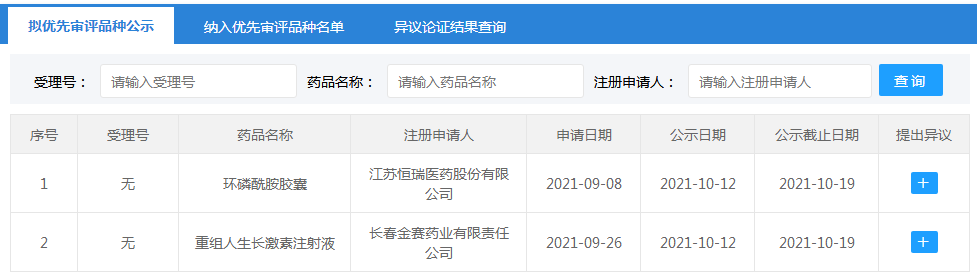
Source: CDE official website
Hengrui Pharmaceutical "Cyclophosphamide Capsules"
Hengrui Medicine's cyclophosphamide capsules are planned to be included in the priority review with "new varieties, dosage forms and specifications of children's drugs that meet the physiological characteristics of children". The proposed indications include: ①Malignant lymphoma (Ann Arbor stage III and IV), Hodgkin’s lymphoma, lymphocytic lymphoma (nodular or diffuse), mixed cell lymphoma, histocytic lymphoma, Burkitt’s lymphoma; ② multiple myeloma; ③ leukemia: chronic lymphocytes Acute leukemia, chronic myelogenous leukemia (acute blast crisis is usually not effective), acute myeloid and monocytic leukemia, acute lymphocytic (stem cell) leukemia (administration of cyclophosphamide during remission can effectively extend the remission period); ④ Mycosis fungoides (advanced); ⑤ Neuroblastoma (diffuse disease); ⑥ Ovarian adenocarcinoma; ⑦ Retinoblastoma; ⑧ Breast cancer. Cyclophosphamide (CTX) is one of the widely used anticancer drugs. Cyclophosphamide for injection was developed by Baxter Oncology GmbH. Hengrui Medicine's cyclophosphamide for injection has passed the quality and efficacy consistency evaluation of generic drugs, and Hengrui Medicine has become the first company to pass the generic drug consistency evaluation. However, no cyclophosphamide capsule dosage form has been approved for marketing.
"Recombinant Human Growth Hormone" of Jinsai Pharmaceutical
Jinsai Pharmaceutical’s recombinant human growth hormone injection is planned to be included in the priority review as "new varieties, dosage forms and specifications of children’s drugs that meet the physiological characteristics of children". The proposed indications include: ①Used for endogenous growth hormone deficiency Slow growth of children caused by; ②for children with short stature caused by Noonan syndrome; ③for children with short stature or growth disorder caused by SHOX gene defects; ④for children with achondroplasia Shortness; ⑤Used for growth disorders in girls caused by gonadal hypoplasia (Turner syndrome); ⑥Used for Prader-Willi syndrome; ⑦Used for adult short bowel syndrome receiving nutritional support; ⑧Used for definite under Growth hormone deficiency caused by thalamic-pituitary disease and significant growth hormone deficiency confirmed by two different growth hormone stimulation tests; ⑨used for the treatment of severe burns. Growth hormone (GH) is a peptide hormone secreted by the anterior pituitary gland of the human body, which can promote the growth of bones, internal organs and the whole body. The recombinant human growth hormone injection developed by Jinsai Pharmaceutical has been approved for 8 indications in China, including the growth disorder of girls caused by gonadal hypoplasia (Turner syndrome), which was just approved at the end of September this year.



