一. In the first half of 2021, the overall situation of drug registration applications
1. Application status of each drug type
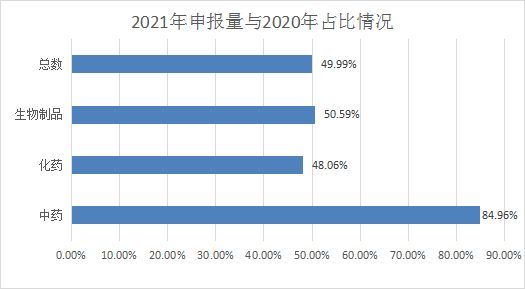
In 2021, CDE has undertaken a total of 5,155 new drug registration applications (calculated by acceptance number, data statistics are as of June 30, 2021, the same below). Compared with 2020, the total number of applications accepted will account for 49.9903% of 2020; 3,800 medicines were accepted, accounting for 48.05868% in 2020; 401 Chinese medicines rose sharply, which was 84.95763% in 2020; 945 biological medicines were not much different from 2020, which was 50.58887%. See Figure 1 and Figure 2 for details of application acceptance in 2021 and comparison with 2020.
Figure 1 Details of declaration in the first half of 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Figure 2 Comparison of application acceptance in 2021 and 2020
Data source: Yaozhi data, Yaozhi consultation and collation
2. Monthly reporting status
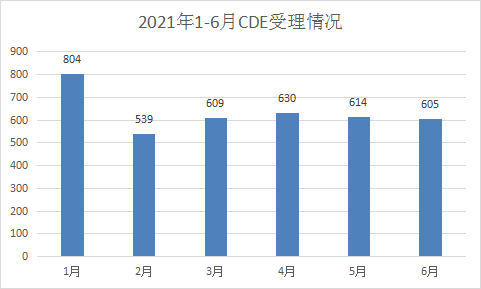
The number of monthly acceptances in the first half of 2021 was basically the same as that of the same period last year, but the June filings were the same as those in March, April, and May, which was significantly less than in June 2020. The monthly acceptance status in the first half of 2021 is shown in Figure 3.
Figure 3 Monthly acceptance in the first half of 2021
Data source: Yaozhi data, Yaozhi consultation and collation
3. Overall situation of various declaration types
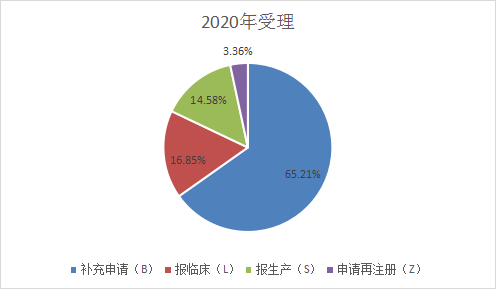
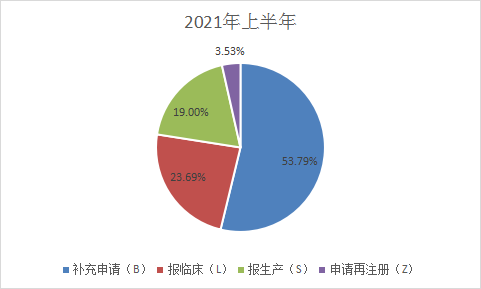
The types of applications in 2021 are: Supplementary applications: 2639, accounting for 51.2% of the total received in 2020, 1162 applications for clinical trials, 932 applications for production, 173 applications for re-registration, as shown in Figure 4 and Figure 5 below, and last year’s Compared with the classification of acceptance numbers, the number of supplementary applications in the first half of 2021 has decreased, and the number of clinical applications and production declarations has increased significantly. Figures 4 and 5 are shown in Figure 4 and Figure 5 for the acceptance of CDE drug application types in 2020 and the first half of 2021.
Figure 4 Classification of filing acceptance in 2020
Data source: Yaozhi data, Yaozhi consultation and collation
Figure 5 Classification of application acceptance from January to June 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Note: Statistical rules: S, L, B, and Z are the 4th letter of the acceptance number respectively
4. Acceptance of domestically produced innovative drugs
From January to June 2021, CDE accepted 622 domestic Class 1 innovative drug registration applications, including 601 clinical applications and 21 marketing applications. According to the statistics of drug types, there are 403 chemical drugs, 193 biological products, and 26 traditional Chinese medicines.
5. Acceptance of imported innovative drugs and original research drugs
From January to June 2021, CDE accepted 73 applications for the registration of imported original research drugs for category 5.1 chemical drugs, and 149 applications for the registration of imported innovative drugs for category 1 drugs.
6. Completion of chemical drug review in the first half of 2021
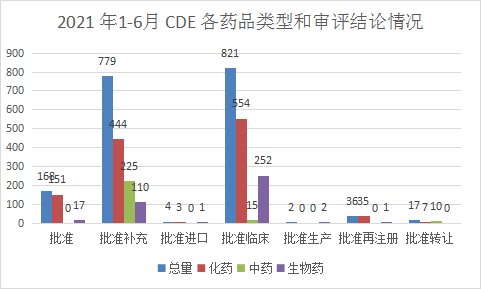
According to the latest statistics from the Yaozhi registration and acceptance database, in 2021 (Note: the date of CDE is from January 1, 2021 to June 30, 2021) the number of acceptance numbers after the review (refers to the review conclusions included in the Yaozhi website) Excluding the acceptance number of the review conclusion but the acceptance number that has not been publicized) totals 1827, including 1194 chemical medicines, 250 traditional Chinese medicines, and 383 biological products. From January to June 2021, the types of CDE drugs and the review conclusions are shown in Figure 6.
Figure 6 CDE drug types and review conclusions from January to June 2021
Data source: Yaozhi data, Yaozhi consultation and collation
7. The situation of the declared area
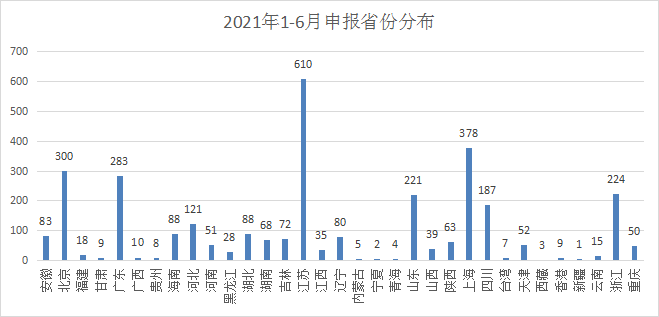
In terms of drug declarations in various provinces and cities, Jiangsu leads with 610 acceptance number, and Shanghai ranks second with 378, followed by Beijing, Guangdong, and Zhejiang. The status of declarations by provinces and cities in 2021 is shown in Figure 7.
Figure 7 Distribution of declarations by provinces 1-6 in 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Let us analyze in detail the registration acceptance and review of chemical drugs, traditional Chinese medicines, biological products, priority review and breakthrough therapy.
二. chemicals
1. The status of chemical drug declarations in the first half of 2021
From January to June 2021, CDE will undertake a total of 3801 new chemical drug registration applications with acceptance numbers. The monthly CDE chemical drug acceptance status in 2021 is shown in Figure 8.
Figure 8 CDE acceptance status from January to June 2021
Data source: Yaozhi data, Yaozhi consultation and collation
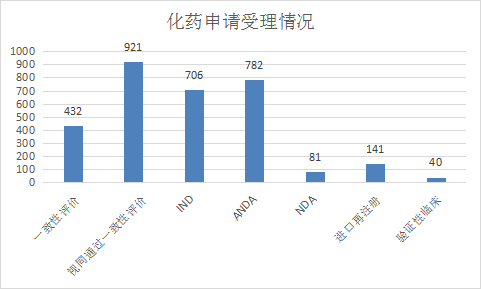
Among the chemical drug registration applications, IND accepted 706 applications, NDA accepted 81 applications, ANDA accepted 782 applications, and accepted 432 applications for consistency evaluation. The acceptance status of various registration applications for chemical drugs from January to June 2021 is shown in Figure 9. .
Figure 9: Acceptance of various registration applications for chemical drugs from January to June 2021
Data source: Yaozhi data, Yaozhi consultation and collation
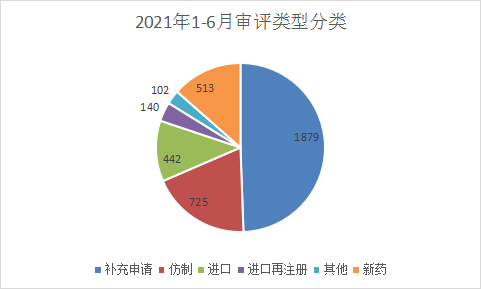
In terms of the types of chemical drug applications, supplementary applications still dominate. In 2021, 1879 chemical drug supplementary applications were accepted for registration, accounting for 49.43% of the total chemical drug applications. Refer to Figure 10 for details of the acceptance status of various application types for CDE chemical drugs in 2021.
Figure 10 Classification of review types from January to June 2021
Data source: Yaozhi data, Yaozhi consultation and collation
1.1 The application status of new chemical drugs in category 1
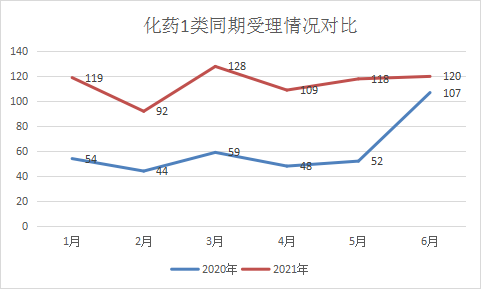
According to the current classification of chemical drug registration, chemical drug category 1 is defined as: innovative drugs that have not been marketed at home or abroad, including drugs that contain new compounds with clear structures and pharmacological effects and have clinical value. From January to June 2021, there were a total of 686 applications for the registration of Class 1 chemical innovative drugs, an increase of 88.46% compared to the same period last year. Among them, 535 were IND applications and 17 were NDA applications. From January to June 2021, monthly chemical drugs were applied. The acceptance status of Class 1 new drugs and the comparison of the same period in 2020 are shown in Figure 11.
Figure 11 Comparison of Class 1 acceptance of chemical drugs in the same period in 2021 and 2020
Data source: Yaozhi data, Yaozhi consultation and collation
1.2 The status of new chemical drugs (domestic) and import declarations of category 1
Among the 686 applications for the registration of category 1 chemical drug innovation drugs, domestic chemical drugs are the main ones. The number of domestic chemical drug innovation drug registration applications is 403 by the acceptance number, and the import chemical drug innovation drug registration application is 149 by the acceptance number. There are 134 supplementary applications for the registration of innovative drugs, including 92 supplementary applications for the registration of innovative drugs for domestic chemical drugs and 42 supplementary applications for the registration of innovative drugs for imported chemical drugs. Refer to Figure 12 for details of the acceptance status of new drugs and import registration applications for chemical drugs in 2021.
Figure 12 The acceptance status of new drugs and import registration applications for category 1 chemical drugs in 2021
Data source: Yaozhi data, Yaozhi consultation and collation
1.3 Acceptance of Consistency Evaluation
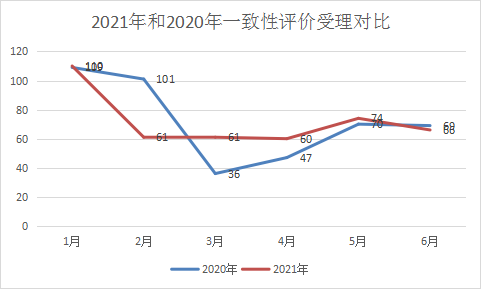
As the consistency evaluation continues, the number of CDE consistency evaluation supplementary acceptances in 2021 and 2020 is the same, both are 432. In the two-year consistency acceptance, January is the peak of acceptance, and 110 in 2021. However, compared with last year, the number of acceptances in February, March, April, May, and June is not much different. The monthly consistency evaluation acceptance in the first half of 2021 and the comparison of consistency evaluation in the same period in 2020 are shown in Figure 13.
Figure 13 Comparison of acceptance of consistency evaluation in 2021 and 2020
Data source: Yaozhi data, Yaozhi consultation and collation
2 Completion of chemical drug review in the first half of 2021
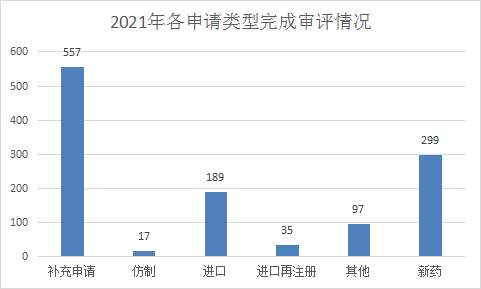
2021 (Note: The CDE undertaking date is from January 1, 2021 to June 30, 2021) CDE completed review (refers to the number of review conclusions included in Yaozhi.com, excluding the end of review, but not publicized The acceptance number of the review conclusion was 1194 chemical drug registration applications, including 557 supplementary applications, 299 new drugs, and 198 imported drugs. Refer to Figure 14 for the complete review of each application type of chemical drugs in the first half of 2021.
Figure 14 Completion of the review of various application types of chemical drugs in the first half of 2021
Data source: Yaozhi data, Yaozhi consultation and collation
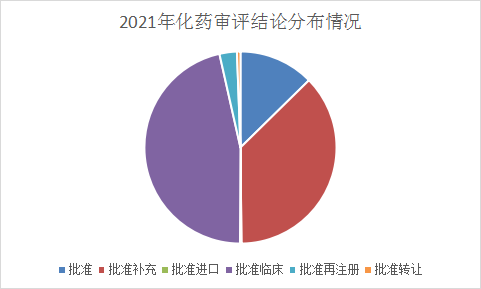
The review conclusions in 2021 will be replaced with approved clinical supplements at most from last year's approval supplements. Approved supplements still occupy a large area of the review conclusions. For specific conclusions, please check the drug registration and acceptance database of Yaozhi.com. Refer to Figure 15 for details of the chemical drug review conclusions from January to June 2021.
Figure 15 The conclusions of the chemical drug review in 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Note: The statistical time is July 05, 2021. Statistics at different time points will cause differences in the number of completed reviews and the number of review conclusions.
二、Chinese medicine
1. Status of Chinese medicine declaration
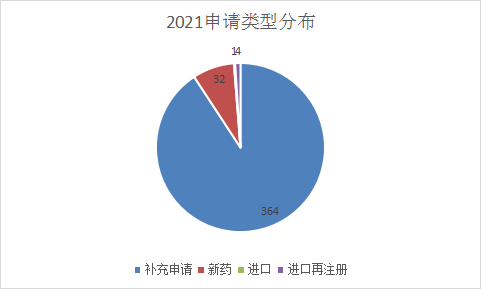
From January to June 2021, CDE will undertake a total of 401 new Chinese medicine registration applications based on acceptance numbers. Affected by the characteristics of its own medicines, Chinese medicines have been in a relatively low state of application. Compared with the same period last year, the number of Chinese medicines accepted has increased significantly compared with the same period last year. This year there are 32 new drug applications, including 25 IND applications and 7 NDAs Among them, new drug applications were mainly applied in January and April, with 10 and 9 respectively. The number of applications in the rest of the month was not much different; supplementary applications still accounted for most of the Chinese medicine acceptance, and 364 applications were accepted this year, and January 2021 Figure 16 shows the comparison between the CDE Chinese medicine acceptance situation in June and the same period in 2020, and the Chinese medicine acceptance situation in 2021 is shown in Figure 17.
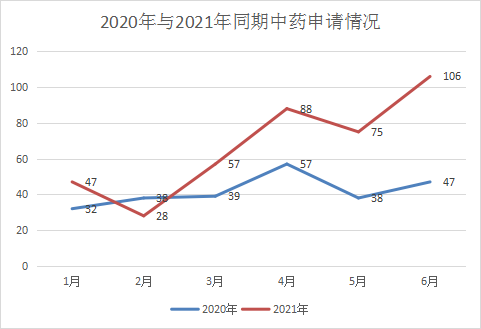
Figure 16 Comparison of TCM applications in 2020 and 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Figure 17 Distribution of Chinese medicine acceptance in 2021
Data source: Yaozhi data, Yaozhi consultation and collation
2. Completion of the review of the application for registration of Chinese medicines
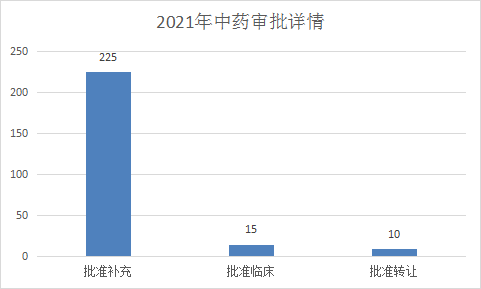
2021 (Note: The CDE undertaking date is from January 1, 2021 to June 30, 2021) CDE completed review (refers to the number of review conclusions included in Yaozhi.com, excluding the end of review, but not publicized The acceptance number of the review conclusion) was 250 TCM registration applications, of which 225 were approved for replenishment, 15 were approved for clinical application (13 new drug IND applications, and 2 were approved for transfer). Figure 18 shows the completion status of the review of individual application types of Chinese medicines in the first half of 2021.
Figure 18 The review completion status of each application type of Chinese medicine from January to June 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Note: The statistical time is July 05, 2021. Statistics at different time points will cause differences in the number of completed reviews and the number of review conclusions.
三、 biological products
1. The status of biological products declaration
From January to June of 2021, CDE will undertake a total of 945 applications for registration of new biological products based on acceptance numbers, accounting for about 50% of the total number of applications accepted last year. Compared with the gradual increase in the number of acceptances in the first half of last year, in 2021, except for January, the number of acceptances in the remaining months has been more stable. The monthly acceptance status of CDE biological products in 2021 and the acceptance status in the same period in 2020 are shown in Figure 19.
Figure 19 Monthly acceptance of CDE biological products in 2021
Data source: Yaozhi data, Yaozhi consultation and collation
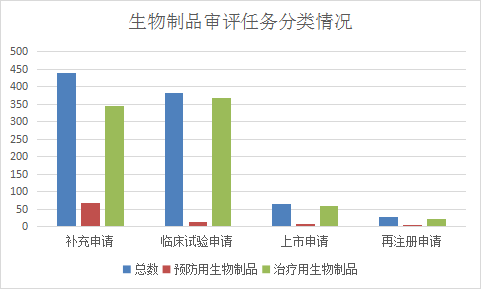
In 2021, CDE will accept 945 applications for registration of biological products (mainly for the application of therapeutic biological products), of which 381 applications for clinical trials of biological products (12 for preventive biological products and 369 for therapeutic biological products) and supplementary biological products will be accepted 438 applications (69 preventive biological products, 344 therapeutic biological products), 65 applications for the marketing of biological products (7 preventive biological products and 58 therapeutic biological products) were accepted. Refer to Figure 20 for details of the acceptance of various registration applications for biological products from January to June 2021.
Figure 20 The acceptance of various registration applications for biological products from January to June 2021
Data source: Yaozhi data, Yaozhi consultation and collation
2. Acceptance of innovative drugs for category 1 biological products
CDE accepted 310 applications for the registration of innovative drugs for category 1 biological products, a significant increase from 336 in the whole year of last year, including 7 biological products for prevention and 303 biological products for treatment. Among the applications for the registration of category 1 biological products, there were 262 applications for clinical trials (6 biological products for preventive use) and 1 application for marketing (biological products for therapeutic use).
3. Completion of the review of the application for registration of biological products
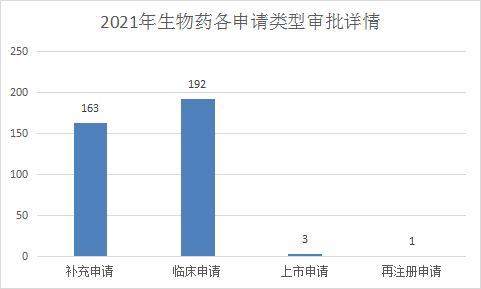
2021 (Note: The CDE undertaking date is from January 1, 2021 to June 30, 2021) CDE completed review (refers to the number of review conclusions included in Yaozhi.com, excluding the end of review, but not publicized There are 382 applications for registration of biological products, including 192 clinical applications (7 preventive biological products and 185 therapeutic biological products) and 163 supplementary applications (17 preventive biological products). And 146 therapeutic biological products). Refer to Figure 22 for details of the review completion status of each application type of biological products from January to June 2021
Figure 21 The review completion status of various application types for biological products from January to June 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Note: The statistical time is July 05, 2021. Statistics at different time points will cause differences in the number of completed reviews and the number of review conclusions.
4. The status of priority review of the inclusion of varietie
In 2021, CDE will include 50 registration applications with acceptance numbers in the priority review process, including 25 chemical drugs, 23 biological products, and 2 traditional Chinese medicines, including 13 children’s drugs, 13 other priority review and approval situations, and qualified approval 12 drugs; indications include idiopathic short stature, psoriasis, cancer, etc.area.
5. Inclusion of breakthrough treatment varieties
Conditions for applying breakthrough therapeutic drug procedures: During the clinical trial of the drug, it is used to prevent and treat diseases that are severely life-threatening or seriously affect the quality of life, and there is no effective prevention and treatment method or there is sufficient evidence to show that it has obvious clinical advantages compared with existing treatment methods. Innovative drugs or improved new drugs. From January to June 2021, CDE will include 26 registration applications (calculated by acceptance number) as breakthrough treatment products. Only Boehringer Ingelheim (China) Investment Co., Ltd. JXHL2100015 has an acceptance number that CDE will accept after 2021-01-01 , The indication is schizophrenia; for details, please check the drug registration and acceptance database of Yaozhi.com.
Data source: Yaozhi drug registration and acceptance database