43 varieties have been reviewed! 10 varieties were the first to be reviewed, Chengdu Better took the lead, Tianjin Hongri, Yichang Renfu...
August 05, 2021
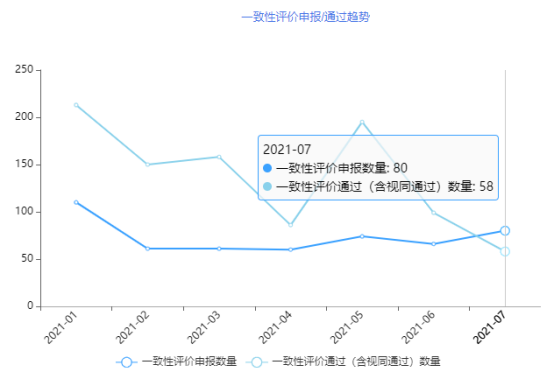
According to the consistency evaluation analysis system of Yaozhi Data Enterprise Edition, 80 new consistency evaluation acceptance numbers were added in July 2021; 58 approvals (including 22 deemed approved approvals) were over-evaluated. (Attached to the end of the article is a detailed form of consistency evaluation of July declaration and over-evaluation)
Figure 1 Trend of filing/approval from January 2021 to July 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Review details
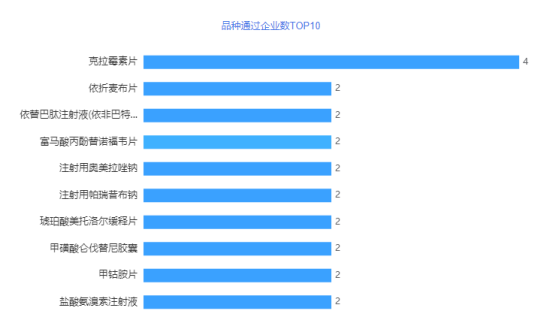
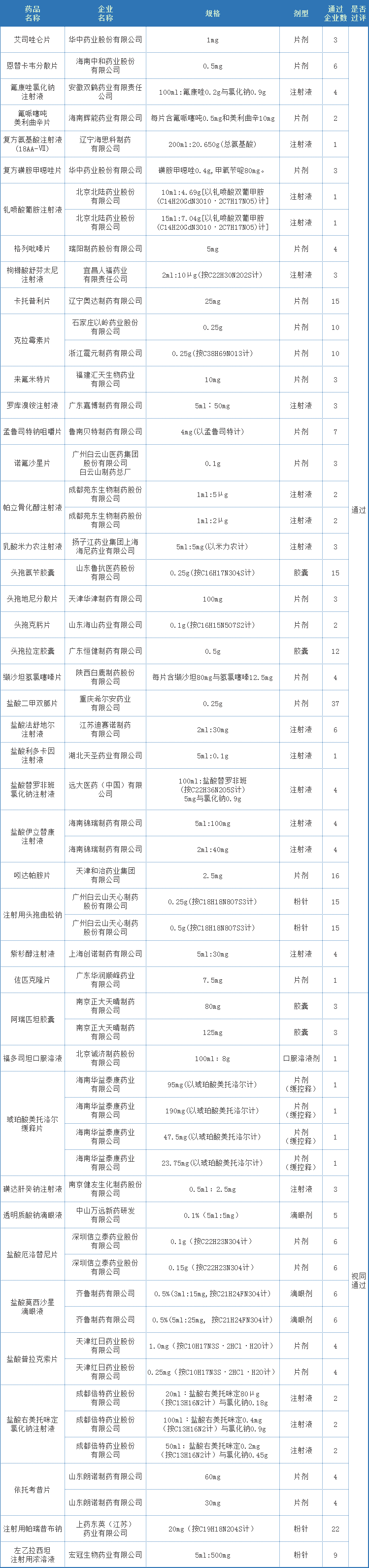
In July, a total of 58 acceptance numbers passed/deemed to pass the consistency evaluation, involving 43 varieties of 43 companies, of which 10 varieties were the first to pass the evaluation. In terms of over-assessment companies, Chengdu Beite ranked first in July, with 3 varieties passing the consistency evaluation. Followed by the six companies including Huazhong Pharmaceutical, Tianjin Hongri, and Yichang Renfu, both of which passed the consistency evaluation. Chengdu Better Pharmaceutical Co., Ltd. is a high-tech enterprise specializing in the research and development, production and sales of high-end generic drugs, innovative drugs, Chinese patent medicines and APIs. After years of innovation and development, it has gradually developed into an innovative pharmaceutical company with advanced R&D concepts, strong R&D strength, complete product pipelines, excellent production quality and a sound marketing network. The company has 308 approval documents in China's listed drug database, involving 182 varieties. The company has currently declared/deemedly declared 66 varieties, and 32 varieties including propofol tenofovir fumarate tablets, levetiracetam injection concentrated solution, and ampicillin sodium for injection have been evaluated (including video Same through). Huazhong Pharmaceutical Co., Ltd. is a modern comprehensive pharmaceutical enterprise integrating R&D, production and sales of pharmaceutical products. The company has strong technical force, complete quality inspection equipment and quality assurance system, advanced production technology and equipment, and has passed GMP, FAMI-QS, HACCP, ISO9001, ISO22000, KOSHER, HALAL and other certifications. The company's pharmaceutical preparations have developed rapidly. It already has nearly a hundred varieties of products such as vitamins, antibiotics, cardiovascular, antipyretic and analgesic products, with a production scale of 10 billion tablets, and sufficient market supply capacity. The company has 186 approvals in China's listed drug database, involving 169 varieties. The company has currently declared/deemedly declared 37 varieties, and 13 varieties including ambroxol hydrochloride injection, compound sulfamethoxazole tablets, and piracetam tablets have been evaluated (including deemed passed). Tianjin Hongri Pharmaceutical Co., Ltd. is a high-tech pharmaceutical and health industry that spans many fields such as finished drugs, traditional Chinese medicine industry clusters, pharmaceutical excipients and raw materials, medical devices, and integrates investment and financing, production, research and development, and sales. Cluster. Relying on the established National Enterprise Technology Center and the National and Local Joint Engineering Research Center for the Key Technology of Chinese Medicine Formula Granules, Hongri Pharmaceutical Co., Ltd. has worked with renowned academicians such as Zhong Nanshan and Zhang Boli to continue to carry out anti-tumor and Xuebijing treatments for new coronary pneumonia, Innovative research on key technologies for the secondary development of Chinese patent medicines. The company has 30 approval documents in China's listed drug database, involving 22 varieties. The company has currently declared/deemedly declared 13 varieties, and 5 varieties including Fasudil Hydrochloride Injection, Pramipexole Hydrochloride Sustained Release Tablets, and Pramipexole Hydrochloride Tablets have been reviewed (including deemed approved).
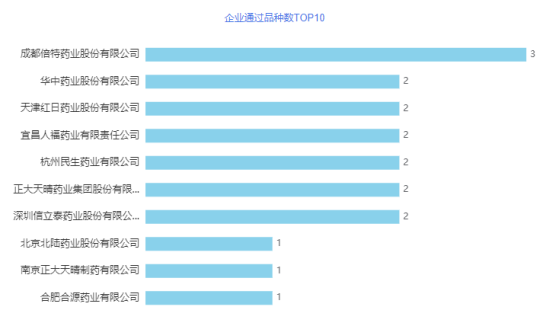
Figure 2 TOP10 products approved by enterprises in July 2021
Data source: Yaozhi data, Yaozhi consultation and collation
In terms of varieties, the most intensely competitive variety in July was clarithromycin tablets, and 4 companies scrambled to comment. In addition, ezetimibe tablets and other varieties have been reviewed by 2 companies. So far, 21 companies have applied for consistency evaluation of clarithromycin tablets, and 9 companies have passed the evaluation. Clarithromycin tablets are suitable for the following infections caused by clarithromycin-sensitive bacteria: 1. Nasopharyngeal infections: tonsillitis, pharyngitis, and paranasal sinusitis; 2. Lower respiratory tract infections: including bronchitis, bacterial pneumonia, and atypical pneumonia; 3. Skin infections, impetigo, erysipelas, folliculitis, boils and wound infections.
Figure 3 TOP 10 companies passing the variety in July 2021
Data source: Yaozhi data, Yaozhi consultation and collation
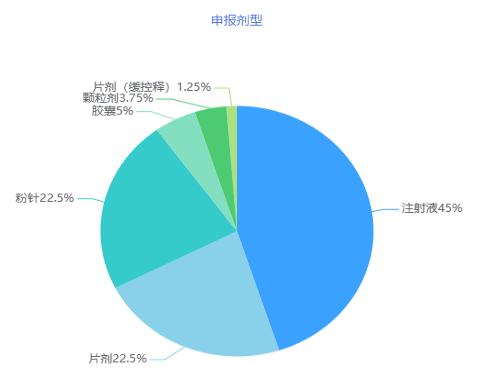
54 varieties are declared, and injections exceed 60%
In July 2021, CDE added 80 acceptance numbers for consistency evaluation, involving 54 varieties of 48 companies, of which injections accounted for more than 60%.
Figure 4 Details of dosage forms declared in July 2021
Data source: Yaozhi data, Yaozhi consultation and collation
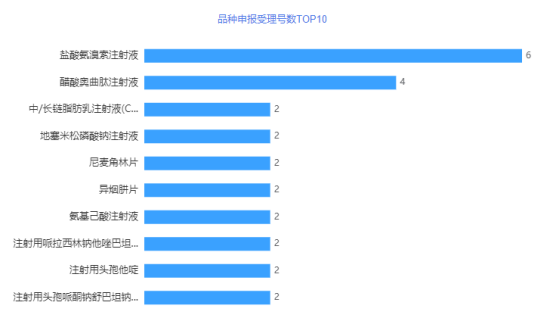
In terms of the declared varieties, in July 2021, 6 acceptance numbers for ambroxol hydrochloride injection were accepted, and 4 acceptance numbers for octreotide acetate injection were accepted. For details, see the figure below.
Figure 5 TOP10 Number of Variety Application Acceptance Numbers in July 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Ambroxol hydrochloride injection is suitable for acute and chronic lung diseases accompanied by abnormal sputum secretion and poor sputum expectoration. For example, the expectorant treatment of acute exacerbation of chronic bronchitis, wheezing bronchitis and bronchial asthma. Preventive treatment of pulmonary complications after surgery. Treatment of infant respiratory distress syndrome (IRDS) in premature infants and newborns. There are 91 approvals for cefuroxime sodium for injection in China, involving 50 companies. At present, 44 companies have applied for consistency evaluation, and 27 companies have passed the evaluation. Octreotide acetate injection is indicated for 1. Emergency treatment of esophageal-gastric varices bleeding caused by liver cirrhosis, combined with special treatment (such as endoscopic sclerosing agent treatment). 2. Relieve symptoms and signs related to gastrointestinal pancreatic endocrine tumors. 3. Prevention of post-pancreatic complications 4. Acromegaly patients who have failed surgery, radiotherapy or dopamine receptor agonist therapy can control symptoms and reduce the concentration of growth hormone and growth hormone mediator C. This product is also suitable for patients with acromegaly who are unable or unwilling to operate, as well as patients with intermittent periods who have failed radiotherapy. There are 27 approvals for octreotide acetate injection in China, involving 17 companies. At present, 11 companies have applied for consistency evaluation, and no company has ever reviewed it. From an enterprise perspective, in July, Hainan Zhonghe Pharmaceutical Co., Ltd. received 4 acceptance numbers for consistency evaluation, ranking first on the list. Shandong Qidu, Shanxi Guorun, and Chengdu Shengnuo Biotechnology have all accepted three acceptance numbers for consistency evaluation. See the figure below for details.
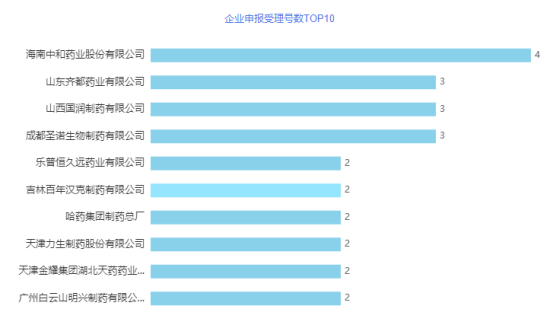
Figure 6 TOP10 number of enterprise application acceptance numbers in July 2021
Data source: Yaozhi data, Yaozhi consultation and collation
Hainan Zhonghe Pharmaceutical Co., Ltd. is an enterprise specializing in the production of peptide drugs. Its production scope includes freeze-dried powder injections, small-volume injections, and bulk drugs. The company has currently declared/deemed to declare 6 varieties, of which Entecavir Dispersible Tablets and Entecavir Capsules have been reviewed.
Attached Table 1: Details of the consistency evaluation passed (including deemed passed) in July 2021
Attached Table 2: Details of the consistency evaluation of the declaration in July 2021
Data as of August 2, 2021
Data source: Yaozhi Data Generic Drug Consistency Evaluation and Analysis System