CAR-T therapy ushered in a harvest period, how to break through the safety bottleneck?
August 12, 2021
Recently, Yaozhi data shows that the listing application of WuXi's Noreki Orencel injection (relma-cel) has entered the "pending approval" status, and it is expected to be approved as the second CAR-T product in China. Previously, Fosun Kate's CAR-T cell therapy product, Akirensai injection (also known as Yiqililunsai injection, code name: FKC876), was the first to be approved for marketing in June 2021. CAR-T cell therapy, as a brand-new cancer treatment method, has attracted much attention from the market. Since 2017, a number of CAR-T cell therapies have been approved for marketing worldwide. However, with the further application of CAR-T therapy, safety issues have become increasingly prominent.
1. CAR-T technology is developing iteratively, but there are still security problems
As early as 40 years ago, scientists learned that T cells play an important role in the fight against cancer. These cells can recognize and eliminate tumor cells, but a small number of T cells in a patient cannot successfully encircle a large number of tumor cells. Subsequently, the basic science has been continuously updated, and tumor immunotherapy around T cells has gradually become a reality. At present, CAR technology has been developed for many generations (Figure 1).
Figure 1. Schematic diagram of the difference between traditional CD8+ T cells and CD8+ CAR-T cells in killing tumor cells and the evolution of CAR structure.
However, the side effects of the approved CAR-T therapy are gradually emerging. When genetically engineered CAR-T cells are reinfused into the patient's body, in addition to their anti-tumor effects, they may also cause cytokine release syndrome (CRS), which seriously threatens the life of the patient. In order to improve the safety of CAR-T therapy, a current solution is to design a fast and reversible "off" or "on" safety switch for CAR-T cells. The optimized CAR-T cells may have better efficacy and fewer side effects. Recently, Southern Medical University Kui Cheng et al. published an article in the Journal of Medicinal Chemistry and summarized some of the current major researches on small molecule-based CAR-T cell new safety switches.
2. Small molecule safety switch: reduce the toxicity related to CAR-T cell therapy
Certain small molecule compounds are expected to give CAR-T cell functional flexibility, allowing it to switch between "on" and "off" states. In addition, they can also be selectively delivered to target tissues using targeted drug delivery technology, which will help to further alleviate the toxicity problem. The small molecule compounds currently reported for CAR-T switch design mainly include FITC, folic acid, rimiducid, rapamycin, proteolytic targeting chimera (PROTAC) compounds, and dasatinib. (figure 2).
Figure 2. Schematic diagram of the different mechanisms of the application of small molecule compounds to safety switches.
2.1 The assembly of immune-like synapses mediated by FITC/folic acid-based safety switches
This is a dual-function/dual-specific safety switch that contains two key modules: one has the ability to specifically recognize and bind to TAA, and the other has the ability to bind to the reprogrammed antigen recognition domain of CAR. This safety switch acts as a pseudo-immune synapse between CAR-T cells and tumor cells in a time- or dose-dependent manner, turning CAR-T cells into an "on" state. Only after the small molecule safety switch is given can it form an immune-like synapse that triggers CAR-T cells to destroy target cancer cells. FITC and folic acid are often used to design such safety switches (Figure 3).
Figure 3. Schematic diagram of safety switch based on FITC/folic acid
FITC is the most widely used fluorescent dye. It has the advantages of high fluorescence quantum yield, susceptibility to changes in the microenvironment without significant impact on the specificity of the conjugated antibody. Moreover, the gene sequence of anti-FITC antibodies has been extensively studied, which has promoted the use of FITC in the design of CAR-T cell safety switches. Folic acid is a water-soluble B vitamin. The differential distribution of FRα in normal tissues and tumor tissues and the high affinity and stability of folic acid make FRα and folic acid a potential safety switch for design.
2.2 Using rapamycin to induce the assembly of FKBP12 and FRB binding domains to control the activation of CAR-T cells
CAR-T cells have an extracellular antigen recognition domain and an intracellular signal domain. Another strategy for designing small molecule-based switches is to split these two structures and reconnect them in the presence of small molecule drugs. Rapamycin and rapalogues can be used as dimerization chemical inducers (CID) to assemble the domains introduced into the CAR structure. Rapamycin occupies two different hydrophobic binding pockets of the two proteins at the same time: the human FK506 binding protein (FKBP12) and the FKBP12-rapamycin binding (FRB) domain of FKBP-rapamycin-related protein (FRAP). Therefore, inserting the FKBP12-rapamycin-FRAP/FRB ternary complex into the CAR structure can constitute a small molecule-based safety switch to control CAR-T cell activities. In the presence of rapamycin, the chimeric antigen receptor performs antigen recognition and signal transduction functions. In the absence of rapamycin, the antigen recognition domain is separated from the signal transduction domain of the CAR structure, leaving CAR-T cells in a "off" state (Figure 4). Therefore, rapamycin can be used to remotely regulate the activities of CAR-T cells to avoid potentially lethal toxic effects.
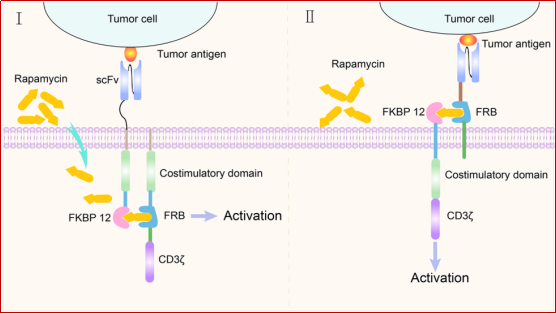
Figure 4. Schematic diagram of rapamycin as a molecular switch
2.3 Rimiducid induces Caspase-9 dimerization and promotes the apoptosis of over-activated CAR-T cells
When the CAR structure contains a fusion protein encoding a suicide gene, CIDs such as Rimiducid (AP1903) and ganciclovir can induce CAR-T cell apoptosis. Using Rimiducid and inducible caspase-9 to form a safety switch can quickly terminate the therapeutic activity of CAR-T cells through apoptosis induced by small molecules (Figure 5).
Figure 5. iCas9 system induces CAR-T cell apoptosis.
2.4 PROTAC technology controls the cracking and degradation of CAR
When CAR recognizes and binds to the relevant tumor antigens on the surface of target tumor cells, CAR-T cells remain activated. Therefore, a safety switch can be designed to remotely and reversibly adjust the surface appearance of CAR by precisely controlling the degradation of CAR, so as to regulate the anti-cancer activity of CAR-T cells and reduce potential toxicity. Currently, PROTAC is the most effective method to degrade specific proteins in cells. CAR is a synthetic protein. By incorporating degrons into CAR, once the degrons are recognized by proteolytic enzymes, it should theoretically be feasible to degrade the entire CAR protein. At present, a variety of new small molecules have been used to control the degradation of CAR (Figure 6).
Figure 6. Schematic diagram of PROTAC technology controlling CAR degradation through the ubiquitin-proteasome system
2.5 Dasatinib directly prevents CAR-T cell activation
Existing drugs can also be used as pharmacological safety switches to temporarily inactivate CAR-T cells to control their toxicity. In addition, the anti-tumor effect can be restored after the safety switch drug is stopped. Dasatinib is a small molecule polytyrosine kinase inhibitor against BCR-ABL and SRC. It inhibits TCR-mediated signal transduction and cell proliferation by inhibiting lymphocyte-specific protein tyrosine kinase (Lck) , Cytokine production and T cell response in vivo. Therefore, Dasatinib can be used as a potential pharmacological safety switch drug. However, Dasatinib is not specifically designed as a small molecule safety switch, and it is still controversial to call it a small molecule safety switch for CAR-T cells.
3. Summary: Small molecule CAR-T switch has a long way to go
The current research on CAR-T cell therapy mainly focuses on improving clinical efficacy, reducing antigen escape and enhancing the specificity of CAR-T cells. In order to achieve these goals, various modifications have been introduced into the chimeric antigen receptor construct. At present, the fourth-generation CAR, which is in the development stage, can deliver cytokines or chemokines to produce resistance to the immunosuppressive tumor microenvironment. In addition, the dual-target and multi-target CARs being developed can simultaneously bind two or more tumor antigens to improve the specificity of tumor cells. Although CAR-T cell therapy has excellent clinical performance, when the tumor load is unpredictable and the activity of T cells is not controlled, it is still difficult to achieve the optimal treatment level without causing serious side effects. Therefore, improving the safety of CAR-T cell therapy is still an important direction for future improvement of the therapy. At present, a variety of small molecule compounds have been reported to be used in CAR-T switch design, but it is still in the early stage of research. It remains to be seen whether the safety of CAR-T therapy can be truly improved.
references
Zheng, Y., Nandakumar, K.S. and Cheng, K. Optimization of CAR-T Cell-Based Therapies Using Small-Molecule-Based Safety Switches. Journal of Medicinal Chemistry. 2021
https://doi-org.ezproxy.lb.polyu.edu.hk/10.1021/acs.jmedchem.0c02054









