Overview of Lung Cancer
(1) Definition of lung cancer
Primary bronchial lung cancer, referred to as lung cancer, originates from the trachea, bronchial mucosa or glands, and is the most common primary malignant tumor of the lung. Early-onset lung cancer is mainly classified by histopathology. Lung cancer can be roughly divided into two categories: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Among them, non-small cell lung cancer accounts for about 80%-85%. , The rest are small cell lung cancer, non-small cell lung cancer mainly includes two subtypes, adenocarcinoma and squamous cell carcinoma. Lung cancer is non-infectious, but has certain family clustering and genetic susceptibility. From the perspective of genotyping, common genotypes include EGFR mutations, ALK mutations, ROS1 mutations, BRAF mutations, and so on.
Figure 1 Histopathological typing and driver genotyping of lung cancer
There are significant differences in the lung cancer driver gene maps between China and Europe and America. In China, EGFR mutations accounted for 50.3%, KRAS mutations and ALK mutations accounted for 6.7% and 4.3%, respectively. In contrast, KRAS in Europe and the United States accounted for a relatively large proportion (25.0%), and EGFR mutations accounted for a relatively small proportion (EGFR sensitizing and EGFR mutations). Other total 29.0%), ALK mutation is slightly higher (7.0%). The EGFR mutation rate in Asian lung cancer patients is significantly higher than that of Europeans and Americans, so EGFR-TKI is called a "gift" from God to Asians.
(2) Causes and clinical symptoms
The etiology of lung cancer has not yet been fully clarified. The main pathogenic factors include smoking, occupational exposure, air pollution, ionizing radiation, diet, genetics, and lung history. The clinical manifestations of lung cancer are diverse but lack specificity, which often leads to delays in the diagnosis of lung cancer. Peripheral lung cancer usually does not show any symptoms, and is often found during physical examinations or chest imaging examinations for other diseases. The clinical manifestations of lung cancer can be summarized as: symptoms caused by the local growth of the primary tumor itself, symptoms caused by the invasion of adjacent organs and structures by the primary tumor, symptoms caused by distant metastasis of the tumor, and extrapulmonary manifestations of lung cancer (paratumor syndrome, side effects) Tumor syndrome] etc.
(3) Classification and staging of diseases
According to the clinical disease progression, the condition of lung cancer patients is staged: small cell lung cancer is divided into two main stages: limited stage and extensive stage; non-small cell lung cancer is divided into stages I, II, III, and IV, of which stage I is an early stage, which means that the tumor is located in the lung tissue. No transfer has occurred. Stage II is a metaphase, which means that cancer cells have spread to the lymph nodes near the hilar. Stage III belongs to the middle and advanced stages, which means that the cancer cells have further metastasized to the mediastinum or extrapulmonary lymph nodes. Stage IV is an advanced stage, which refers to the occurrence of pleural metastasis, pleural effusion, or multiple metastases throughout the body, such as liver, brain, and bone.
Disease Epidemiology Research
A large number of epidemiological studies have confirmed that smoking is the biggest risk factor for lung cancer. There are more than 50 substances in tobacco that can cause cancer. 70% to 80% of lung cancers are related to smoking (including passive smoking). With age, lung cancer develops. The probability of being diagnosed with lung cancer will increase. Half of lung cancer patients are over 70 years old. In addition, people with a history of cancer, long-term exposure to carcinogens (radon, arsenic, soot, silicon dioxide, etc.), and other lungs Patients with diseases (such as COPD, pulmonary fibrosis) have an increased risk of lung cancer. According to CA Cancer J Clin, there were approximately 18.1 million new cancer cases and 9.6 million cancer deaths worldwide in 2018. Lung cancer is still the number one tumor in incidence (11.6%) and death (18.4%), with 209.4 new cases. Million and 1.761 million deaths. In terms of regions, Asia has the highest proportion of new lung cancer cases (48.4%) and deaths (57.3%) in the world, with China accounting for the highest proportion.
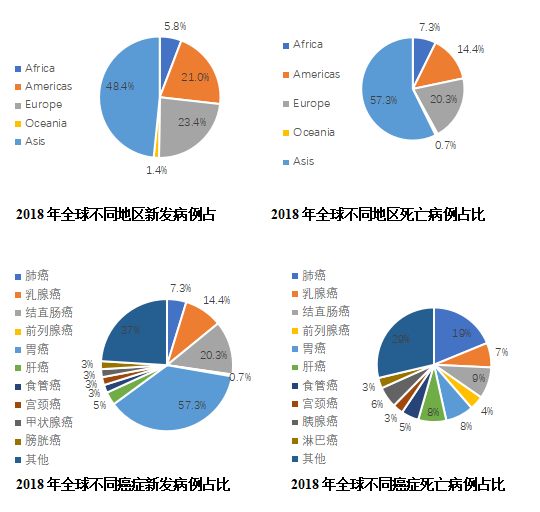
Figure 2 2018 global cancer epidemiology pie chart
According to the 2018 analysis report of the International Agency for Research on Cancer, the incidence of lung cancer was 22.48 per 100,000 (2.094 million new patients per year), and the mortality rate was 18.51 per 100,000 (1.761 million new deaths per year). Both morbidity and mortality were In the first place. According to data from the American Cancer Center website, the incidence of lung cancer in the United States is 60.5 per 100,000, and the mortality rate is 41.9 per 100,000. The incidence rate is second, and the mortality rate is the first. According to China's cancer statistics, the incidence of lung cancer in China is 57.13/100,000, and the mortality rate is 45.8/100,000, both of which rank first among all cancers.
Figure 3 Comparison of lung cancer incidence and mortality in China and the United States (cases per 100,000)
The 5-year survival rate of lung cancer in China is low. According to data published in The Lancet in 2018, from 2010 to 2014, the 5-year survival rate of lung cancer in Japan was as high as 32.9%, the 5-year survival rate of lung cancer in the United States was 21.2%, and that in China was 19.8% (slightly higher than pancreatic cancer and liver cancer) Although the survival rate of lung cancer in China is slightly higher than that of European countries such as Germany, France, and the United Kingdom, it is lower than that of Japan and the United States. There are also significant differences in survival rates compared with other cancer types.
Figure 4 Five-year survival rate of lung cancer in China, the United States, Germany, France, and the United Kingdom from 2000 to 2014 (%)
Disease treatment analysis
3.1 Diagnosis and treatment of lung cancer
(1) Screening and diagnosis of lung cancer
Screening of high-risk populations. Carrying out lung cancer screening in high-risk populations is beneficial to early detection of early lung cancer and improve the cure rate. Low-dose spiral CT (low dose computedtomography (LDCT)) is 4 to 10 times more sensitive to the detection of early lung cancer than conventional chest X-rays, and can detect early peripheral lung cancer early. According to data from the International Early Lung Cancer Action Plan, LDCT annual screening can detect 85% of peripheral lung cancers, and the 10-year expected survival rate after surgery is 92%.
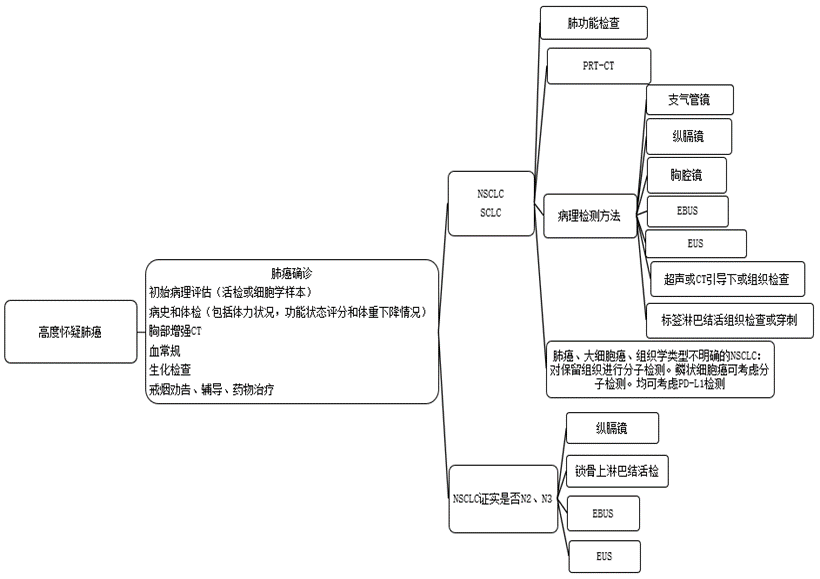
Figure 5 Flow chart of lung cancer biopsy
1) Imaging examination
Chest X-ray: It can understand the location of lung cancer, the size of the invasion of neighboring parts, and the accompanying inflammatory lesions. It is an important means for early detection of lung cancer. "S-shaped reflection" (or "anti-S sign") is a typical sign for the diagnosis of lung cancer. Computed tomography (CT): Chest CT can further verify the location and scope of the lesion, and can also roughly distinguish between benign and malignant. It is currently an important means of diagnosing lung cancer. Magnetic resonance imaging (MRI), B-ultrasound, emission computed tomography (ECT), positron emission computed tomography (PET-CT), etc., are especially suitable for judging the metastasis of lung cancer in the brain, lymph nodes, bones and other tissues. Endoscopy can directly observe the lesions and obtain the tissues or cells of the lesions, which is convenient for pathological diagnosis. Bronchoscopy is one of the main methods of diagnosing lung cancer. It can directly observe the lesions in the bronchus. It is mainly suitable for central lung cancer. When necessary, ultrasound-guided mediastinal lymph node biopsy (EBUS) can be used to confirm the pathological diagnosis.
Mediastinoscopy is currently the gold standard for clinical evaluation of the status of mediastinal lymph nodes in lung cancer. It is mainly used for cases with mediastinal lymph node metastasis, which are not suitable for surgical treatment and cannot be diagnosed by other methods. Thoracoscopy is used to perform lesion resection and examination of small lung lesions, suspicious lymph nodes, pleura, pericardium and other tissues under thoracoscopy. It can accurately diagnose and clinically stage lung cancer. It is suitable for bronchoscopy and percutaneous intrapulmonary lesion puncture. Biopsy cannot obtain pathological specimens or the diagnosis of combined pleural lesions.
2) Pathological examination
Cytological examination: Cells obtained from endoscopy, fine-needle aspiration, pleural effusion, and sputum can be subjected to cytological examination to make a preliminary diagnosis. Histological examination: Examination of lung biopsy is the gold standard for the diagnosis of lung cancer. Genetic examination: genetic examination of tumor tissues, such as EGFR gene mutation, ALK and ROS1 gene fusion detection, etc., are conducive to individualized targeted therapy. Laboratory tests: blood routine, liver and kidney function and other necessary biochemical and immunological tests, blood coagulation function tests, etc., to facilitate the evaluation of the patient's overall condition before and after surgery. Tumor markers such as carcinoembryonic antigen (CEA), neurospecific enolase (NSE), cytokeratin 19 fragment antigen (CYFRA21-1), gastrin releasing peptide precursor (ProGRP), squamous cell carcinoma antigen ( SCC-Ag) and other joint examinations have certain reference value for the diagnosis of lung cancer.
(2) Treatment of lung cancer
Figure 6 Overview of domestic and foreign treatment guidelines for lung cancer
The treatment of lung cancer should be based on the combination of multidisciplinary team (MDT) and individualized treatment, that is, according to the patient’s physical condition, tumor’s histopathological type and molecular type, invasion range and progression trend. Disciplinary comprehensive treatment model, planned and reasonable application of surgery, radiotherapy, chemotherapy, molecular targeted therapy and immunotherapy, etc., in order to maximize the survival time of patients, increase survival rate, manipulate tumor progression and improve patients’ health Quality of Life.
1) Surgical treatment
Anatomical pneumonectomy is an important treatment for early and mid-stage lung cancer, and it is also an important method for clinical cure of lung cancer. Lung cancer surgery is divided into complete resection, incomplete resection and indeterminate resection. Strive for complete resection, in order to achieve complete tumor resection, reduce tumor metastasis and recurrence, and at the same time carry out precise pathological TNM staging, strive for molecular pathological classification, and guide comprehensive postoperative treatment.
2) Radiotherapy
Radiotherapy for lung cancer includes radical radiotherapy, palliative radiotherapy, adjuvant radiotherapy and preventive radiotherapy.
Table 1 Radiotherapy for lung cancer
3) Medication
Drug treatments for lung cancer include chemotherapy, molecular targeted therapy, and immunotherapy. Chemotherapy is divided into neoadjuvant chemotherapy, adjuvant chemotherapy, and palliative chemotherapy. The clinical indications should be strictly controlled and implemented under the guidance of oncologists. Drug treatments for lung cancer include chemotherapy, molecular targeted therapy, and immunotherapy. Chemotherapy is divided into neoadjuvant chemotherapy, adjuvant chemotherapy, and palliative chemotherapy. The clinical indications should be strictly controlled and implemented under the guidance of oncologists. Chemotherapy should fully consider the patient's disease stage, physical status, adverse reactions, quality of life and patient wishes, and avoid over-treatment or under-treatment. The efficacy of chemotherapy should be evaluated in a timely manner, and adverse reactions should be closely monitored and prevented, and drugs and (or) doses should be adjusted as appropriate. Molecular targeted therapy needs to clarify gene mutation status and guide targeted therapy based on molecular classification. In recent years, immunotherapy represented by immune checkpoint inhibitors (such as PD-1 monoclonal antibody or PD-L1 monoclonal antibody, etc.) has made gratifying progress. Based on the proven survival benefits of immune checkpoint inhibitors and the significant survival benefits proven in the Chinese population, the first domestic PD-1 inhibitor nivolumab [Nivolumab] has been approved for marketing Driver gene-negative advanced NSCLC patients.
Drug treatment of advanced NSCLC
First-line drug treatment: The platinum-containing two-drug regimen is a standard first-line chemotherapy regimen, which can be combined with endostatin on the basis of chemotherapy; for patients with advanced non-driver gene, non-squamous NSCLC, bevacizumab can also be combined on the basis of chemotherapy ; Patients with positive lung cancer driver genes, such as EGFR gene mutations (including 19 exon deletion, 21 exon L858R and L861Q, 18 exon G719X, and 20 exon S768I) positive patients can choose epidermal growth factor Receptor tyrosine kinase inhibitor [EGFR-TKI] treatment, including gefitinib, erlotinib, icotinib, or afatinib treatment, and combination therapy may also be considered when first-line gefitinib treatment is given. Metrexed and carboplatin. Patients with non-small cell lung cancer who are positive for ALK or ROS1 fusion gene can choose crizotinib for treatment. The currently available therapeutic drugs are shown in Table 2 and Table 3.
Table 2 Commonly used first-line chemotherapy regimens for non-small cell lung cancer
Table 3 Anti-angiogenesis drugs and targeted therapy drugs commonly used in non-small cell lung cancer
For patients who have achieved disease manipulation (complete remission, partial remission, and stability) in first-line treatment, maintenance therapy can be selected. At present, the drugs supported by evidence-based medical evidence for maintenance treatment of the same drug include pemetrexed (non-squamous cell carcinoma), bevacizumab (non-squamous cell carcinoma) and gemcitabine; drugs that are supported by evidence-based medical evidence to change the drug for maintenance treatment There is pemetrexed (non-squamous cell carcinoma). Patients with sensitive mutations in the EGFR gene can choose EGFR-TKI for maintenance treatment. Second-line drug therapy: The drugs of choice for second-line therapy include docetaxel, pemetrexed, Nivolumab, EGFR-TKI and crizotinib. For patients with positive lung cancer driver gene mutations, if the corresponding molecular targeted drugs are not used in the first-line and maintenance treatments, molecular targeted drugs should be given priority in the second-line treatment; patients with resistance to EGFR-TKIs and positive EGFRT790M mutation after the first-line EGFR-TKIs treatment, the second-line Osimertinib should be given priority in treatment. Regarding ALK-positive patients, the first-line agrees that patients who develop resistance after crizotinib treatment can be treated with ceritinib sequentially in the second-line treatment. Regarding first-line consent to EGFR-TKI or crizotinib treatment, patients who appear to be resistant to treatment and second-line consent to chemotherapy can choose platinum-containing dual-drug or single-drug treatment based on the patient's ECOGPS score. For patients with negative driver genes, chemotherapy should be given priority. For patients without driver genes and histological type of squamous cell carcinoma, afatinib can be used. For NSCLC patients who have failed platinum-containing two-drug combination chemotherapy/targeted therapy, the PD-1 inhibitor Nivolumab can be selected.Third-line drug therapy: You can choose to participate in clinical trials, and you can also choose oral VEGFR-TKI as a single agent for third-line therapy. At present, anlotinib is supported by evidence-based medicine in the third-line treatment of VEGFR-TKI.
Table 4 Common second-line treatment options for non-small cell lung cancer
Drug therapy for non-resectable NSCLC includes radiotherapy and chemotherapy combined, and simultaneous or sequential radiotherapy and chemotherapy can be selected according to the specific situation. Simultaneous treatment recommended chemotherapy drugs are etoposide combined with cisplatin (EP) or carboplatin (EC), pemetrexed combined with cisplatin or carboplatin, paclitaxel or docetaxel combined with platinum. Sequential governanceThe chemotherapy drugs are cisplatin + etoposide, cisplatin + paclitaxel, cisplatin + docetaxel, cisplatin or carboplatin + pemetrexed [non-squamous non-small cell lung cancer]. The multidisciplinary team discusses and evaluates the possibility of surgery for down-stage patients after induction therapy. If complete resection can be achieved, surgical treatment may be considered.
Perioperative drug treatment of NSCLC
Postoperative adjuvant chemotherapy: completely resected stage Ⅱ~Ⅲ NSCLC, recommended 4 cycles of postoperative adjuvant chemotherapy with platinum two-drug regimen. Adjuvant chemotherapy starts when the patient's physical condition almost returns to normal after surgery, usually 4 to 6 weeks after surgery, and no more than 3 months after surgery is recommended. Neoadjuvant chemotherapy: For resectable stage III NSCLC, you can choose platinum-containing dual-drug, 2 to 3 cycles of preoperative neoadjuvant chemotherapy. The curative effect should be evaluated in time, and adverse reactions should be monitored and dealt with to avoid increasing surgical complications. Surgery is usually performed 2 to 4 weeks after the end of chemotherapy. Postoperative adjuvant chemotherapy should be based on the preoperative staging and neoadjuvant chemotherapy efficacy. The effective one should continue the original regimen or the patient’s tolerance should be adjusted as appropriate, and the treatment regimen should be adjusted for the ineffective. A total of 4 cycles of perioperative chemotherapy are recommended.
Drug treatment of SCLC
First-line treatment plan: T1~2N0 limited-stage small cell lung cancer recommended lobectomy + hilar and mediastinal lymph node dissection, postoperative adjuvant chemotherapy. For patients with limited-stage small cell lung cancer exceeding T1~2N0, comprehensive treatment based on radiotherapy and chemotherapy is recommended. The chemotherapy regimen recommends etoposide combined with cisplatin [EP] or etoposide combined with carboplatin [EC]. Comprehensive treatment based on chemotherapy is recommended for extensive-stage small cell lung cancer, and for those with local symptoms or with brain metastases, combined radiotherapy or other treatment methods on the basis of chemotherapy are recommended. Chemotherapy regimens include EP, EC, irinotecan combined with cisplatin (IP), irinotecan combined with carboplatin (IC), or etoposide combined with loplatin (EL). 〔2〕Second-line treatment plan: Topotecan, irinotecan, gemcitabine, temozolomide, or tax is recommended for those who relapse or progress within 3 months after first-line chemotherapy; topotecan is recommended for those who relapse or progress within 3-6 months , Irinotecan, gemcitabine, docetaxel, temozolomide, or vinorelbine; the initial treatment plan can be selected for those who relapse or progress after 6 months. Encourage patients to participate in clinical trials of new drugs.
4) Bronchoscopy interventional therapy
With the increasing popularity of bronchoscopy in clinical applications, the following local treatments can be used as treatment options for patients who cannot be operated or radiotherapy. Various bronchoscope-mediated lasers, high-frequency electrosurgical units, radiofrequency ablation, and argon plasma coagulation [Argonplasmacoagulation, APC], microwave, laser, photodynamic therapy, freezing, airway stents, balloon dilatation, submucosal or intratumoral drug injection, etc., the implementation of intrabronchial interventional therapy must strictly grasp the indications and clarify the purpose of treatment. Objectively evaluate whether a certain treatment technology to be adopted can achieve the expected goal, and carry out treatment in qualified hospitals.
3.2 Targeted therapy
(1) EGFR: target and TKIs principle of action
EGFR is a member of the epidermal growth factor receptor (HER) family. It is mainly composed of three parts: extracellular domain, transmembrane domain, and intracellular domain tyrosinase. When it binds to extracellular epidermal growth factor (EGF), it will Form a dimer structure to activate downstream signaling pathways. Overexpression of EGFR will activate downstream signaling pathways, making cell growth unable to be inhibited, and enhancing tumor cell proliferation and metastasis, and ultimately promoting tumor occurrence and progression. Receptors bind to ligands, and bind to ligands such as EGF and TGFα. After the body is activated, a dimer is formed. After the intracellular tyrosine kinases are mutually phosphorylated, the phosphorylated tyrosine site binds to the intracellular signal transduction protein to form a signal transduction protein complex, and the signal transduction protein is activated at the same time . The continuously activated EGFR pathway will transmit growth, proliferation and anti-apoptotic signals to tumor cells. Downstream signaling pathways include Ras-MAPK (promote gene transcription, cell division and cell cycle progression), PI3K/Akt (anti-apoptotic pathway and neovascularization), JAK/STAT (cell proliferation and prolong cell survival), PLCγ/PKC ( Cell differentiation, apoptosis, etc.) etc. EGFR-TKIs inhibit downstream signal transmission by inhibiting the phosphorylation of intracellular tyrosine kinase. Small molecule inhibitors block the binding of tyrosine kinase to ATP by competing with ATP to bind the target, thereby affecting the activation of downstream pathways and inhibiting tumor cells.
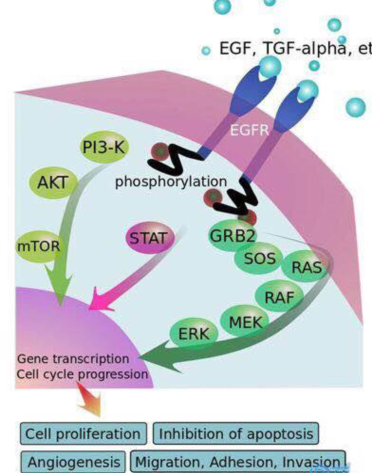
Figure 7 EGFR signaling pathway
(2) EGFR: 19del and L858R are common mutation sites
The common mutation sites of EGFR gene occur in exons 18, 19, 20 and 21. According to nucleotide changes, EGFR mutations can be divided into three categories: the deletion mutations from E746 to S752 encoded by exon 19 are represented; the replacement of a single nucleotide; and insertion or repetitive mutations. Among all the mutation types, 85%-90% are the 19 exon deletion mutation (19del) and the 21 exon L858 point mutation (L858R). Both mutations will cause the activation of the tyrosine kinase domain (and the The sensitivity of EGFR-TKI is related), so these two mutations are called EGFR sensitive mutations. The remaining uncommon mutations that are also sensitive to EGFR-TKI include exon 19 insertion mutation, exon 20 insertion mutation A763_Y764insFQEA, L861Q, G719S and S768I. EGFR gene mutation is an important target for predicting the efficacy of EGFR-TKIs in patients with NSCLC. There are a variety of targeted drugs on the market for EGFR gene mutations, and different EGFR mutation states correspond to different targeted drug choices.
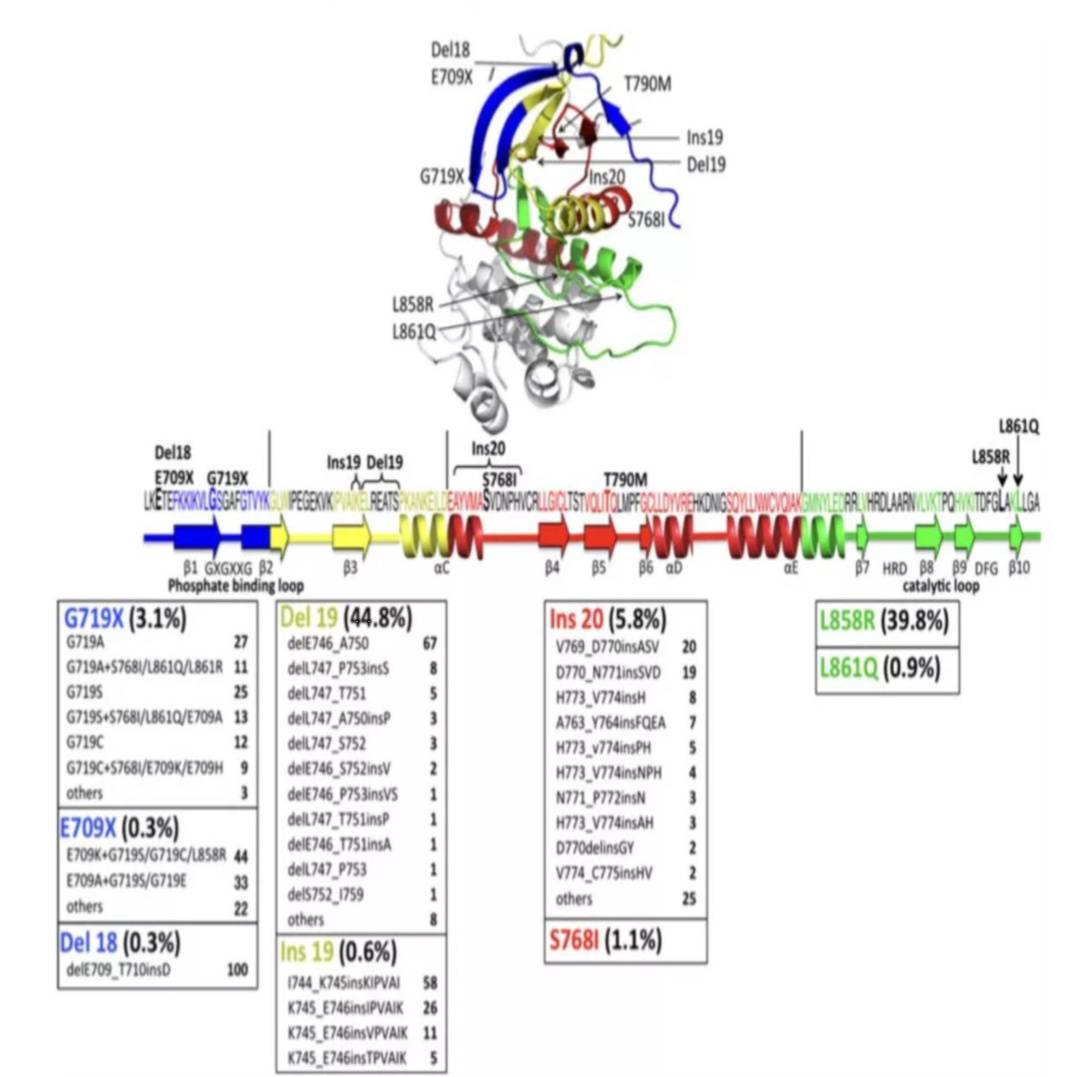
Figure 8 Common mutation sites of EGFR gene
(3) Positive treatment for EGFR sensitive mutations
NCCN treatment guidelines require patients to undergo pathological typing and molecular testing before receiving treatment to determine the pathological type (squamous cell carcinoma or non-squamous cell carcinoma) and gene mutation type of specific NSCLC; 2020V3 guidelines list osimertinib as an EGFR sensitive mutation First-line drugs are the first choice. Erlotinib, afatinib, gefitinib and dacomitinib are recommended as other first-line drugs. If T790M resistance mutations (approximately 60%) are found in treatment, you need to choose Aoshi If tinib continues to be treated, if the small molecule inhibitor is still ineffective after treatment, consider using immunosuppressive agents (first choice) or other chemotherapeutic drugs.
Table 5 NCCN recommends medication for EGFR mutation-positive patients
Gefitinib, erlotinib, and icotinib are the first-generation targeted drugs for lung cancer. They are not firmly bound to the target and separate after a period of time because they are also called reversible targeted drugs. Afatinib and dacomitinib are second-generation targeted drugs that can irreversibly bind to the target and lock the target permanently. Most of the patients receiving first-generation and second-generation EGFR-TKI drug treatments develop resistance within one year of treatment. The reason is that the patient has a T790M mutation. As a third-generation targeted drug, osimertinib can act on the first or second-generation target Specific gene mutations (T790M) that are resistant to drugs have a high degree of selectivity for specific gene mutations.
Source of data chart: public information & yaozhi consulting collation















